
This is an abridged version of a story that first appeared under the same title in Stainless Steel Focus No. 07/2012.
The Nickel Institute's director of promotion, Peter Cutler, and consultant Gary Coates, reveal some of the reasons for the continuing popularity of nickel in stainless steels.
Stainless steel is everywhere in our world and contributes to all aspects of our lives. We find stainless steel in our homes, in our buildings and offices, in the vehicles we travel in and in every imaginable industrial sector. Yet the first patents for stainless steel were issued only 100 years ago.
How did this metal become so desirable over the past century that more than 32 million tonnes was produced in 2011? And how does nickel, a vital alloying element in most stainless steel alloys, contribute to the high demand for stainless steel?
THE 'CREATION' OF STAINLESS STEEL
By definition, a ‘stainless’ steel has a minimum level of about 10.5% chromium, so the discovery of chromium in 1799 by Nicolas Louis Vauquelin in France was the first key event in the creation of stainless steel. In 1821 another Frenchman, Pierre Berthier, published research that showed a correlation between increasing chromium content and increasing corrosion resistance, but the high carbon content of his alloys prevented them from showing a true ‘stainless’ behaviour.
Still in France, in 1904 Leon Guillet first published his metallographic work on alloys that today would be classified as ferritic and martensitic stainless steel. In 1906 Guillet published his work on the nickel-containing austenitic stainless steel family, but his studies did not include corrosion resistance. Albert Portevin then continued to build on Guillet’s work.
In 1911, a German scientist named Philip Monnartz reported that as the chromium content neared 12% in a steel with a relatively low carbon content, the alloy exhibited ‘stainless’ properties. Further developments then rapidly occurred in many other countries. In the United States, Elwood Haynes started working with martensitic alloys while Becket and Dantsizen were developing a ferritic stainless steel as lead-in wires for electric light bulbs. In 1912, Great Britain’s Harry Brearley worked on a 13% chromium martensitic alloy, initially for high temperature service in exhaust valves for aeroplane engines.
Meanwhile in Germany, Eduard Maurer and Benno Strauss were testing nickel-containingalloys and, in 1912, two patents were awarded. One of these grades, containing about 20% chromium and 7% nickel, was called V2A, and was found to have exceptional corrosion resistance in nitric acid. That grade had a relatively high carbon content compared to today’s stainless steel, and would be
similar to a Type 302 (EN 1.4317) stainless steel. 100 years later, the most commonly used alloy for nitric acid is 304L (EN 1.4307) with approximately 18.5% chromium and 8.5% nickel, quite similar to the V2A composition other than having a much lower carbon content.
Brearley’s martensitic stainless steel alloy would not rust when wet. He worked with Sheffield cutlery manufacturers to forge it into knife blades and then harden it, replacing the carbon steel blades they were then making. Stainless steel knives rapidly became a common household item. However, for forks and spoons, where high hardness was not so important, the 18-8 (302) composition became the most commonly used alloy.
300 SERIES
We normally think of the austenitic or 300 series family of stainless steels as the ‘nickel stainless steels’, but many other families contain nickel. One of the prime reasons for using nickel in the 300 series alloys is that nickel is an austenite former, but other reasons include:
- Nickel adds corrosion resistance, especially in certain aqueous environments, and in certain high temperature environments.
- Nickel can retard the formation of embrittling intermetallic phases at elevated temperatures, a major downfall of the non-austenitic families.
- The austenitic structure can mean high toughness at cryogenic temperatures.
- The advantages of the 300 series extend to welding and forming operations.
A fuller discussion of these topics can be found in 'The Nickel Advantage - Nickel in Stainless Steels', available on the Nickel Institute website.
200 SERIES
The 200 series stainless steels are also austenitic in structure. The standardised 200 series grades, which have chromium contents close to the level of a 304L alloy (about 18%), have an intermediate level of nickel. The ‘non-standardised’ 200 series not only have lower contents of nickel, but also lower contents of chromium, with the net effect of significantly reduced corrosion resistance, although still an improvement over the 11-13% chromium ferritic stainless steels.
DUPLEX
The duplex (austenitic-ferritic) family of alloys also need some nickel as well as nitrogen to ensure proper austenite formation. Most ‘matching’ duplex filler metals are actually over-alloyed with nickel to ensure that the welds have the required properties.
PRECIPITATION HARDENABLE
The precipitation hardenable (PH) stainless steel family contain nickel, which increases their corrosion resistance, ductility and weldability compared with hardenable non-nickel-containing stainless steel alloys. One of the other major advantages of the PH grades is that, unlike the martensitic grades, they do not need a quenching operation, which considerably reduces risk of distortion. Some of the martensitic grades also contain a small nickel addition. In the higher chromium types, the nickel is needed for the martensitic transition. In all nickel containing martensitic grades, nickel improves their corrosion resistance, ductility and weldability.
Some of the lower alloyed ferritic grades such as UNS S41003 (EN 1.4001) and S40975 contain a small intentional nickel alloying addition that allows for grain size control, which aids especially in welded constructions. A few of the higher alloyed ferritic grades also have a small nickel addition to increase toughness and ductility, which is beneficial during both hot rolling and in their end use.
Clearly, it is important for each specific application to select the appropriate alloy or alloys to give the desired properties.
GROWTH IN DEMAND FOR STAINLESS STEEL
According to the ISSF, 300 series stainless steel still dominates the worldwide production figures, as shown in Figure 1.
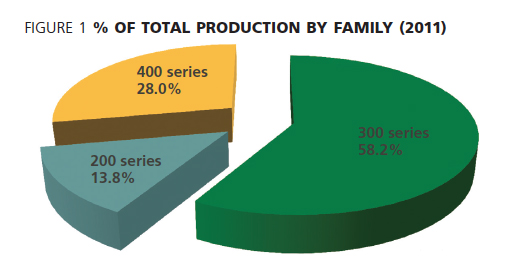
The properties of the various 300 series grades - created by the addition of nickel - are clearly valued by users, both in industry and the general public. Upwards of two thirds of all stainless steel produced in 2011 fell within the 300 series and close to three quarters of all stainless steel produced contains nickel.
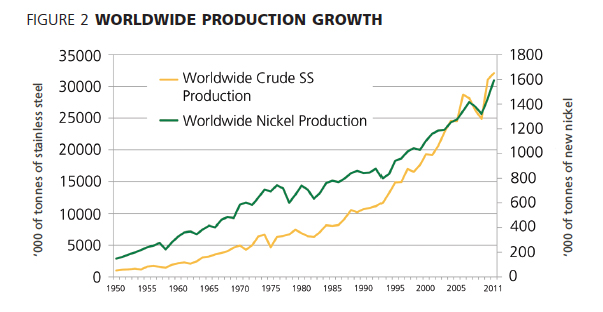
The growth of worldwide production of stainless steel over the past 100 years has been steady, if not spectacular. This has meant that the demand for new nickel has steadily increased along with the demand for stainless steel, as shown in Figure 2. Recycled stainless steel is also a very important component in the alloy supply chain.
EFFICIENCY AND 'GREEN' CREDENTIALS
Resource efficiency is a recurring theme as the global economy faces economic challenges. Stainless steel not only contributes towards efficiency in many applications, it also shows continuous improvement in the resource efficiency related to stainless steel itself.
There are three important factors:
- Stainless steel’s long service life, which might average 15 to 20 years, although much longer in prestigious buildings.
- The extent of recycling: The percentage recovered and recycled at end-of-life - around 90% - is amongst the highest of all materials. Moreover, this recycling can be repeated many times without loss of quality. While the recycled content may appear to be relatively low, this is simply a result of stainless steel’s long service life (15 to 20 years) coupled with much lower global production 15 to 20 years ago.
- Continual production improvements for stainless steel and its raw materials. For example, whilst the ores being processed today are of lower grade than before, the extraction and recovery processes are more efficient.
THE FUTURE
The history of stainless steel would be incomplete without celebrating the extent to which it has enabled innovation not just in the area of improved performance, but also in the more intangible, aesthetic aspects. From chemical plants to medical equipment to iconic stainless steel-clad buildings, stainless steel has made - and will continue to make - a major contribution to almost every aspect of our lives.
With durability, recyclability, versatility and aesthetic appeal at the core of its appeal, stainless steel - with nickel as one of its trusted alloys - is well placed to continue to innovate and expand its applications.
STAINLESS STEEL IN USE
FOOD AND BEVERAGE INDUSTRY
The popularity of stainless steels in kitchens did not go unnoticed in the food and beverage industry.
If we take milk, we know of an early stainless steel bulk milk tank truck from 1927 in the USA. A paper entitled ‘The Corrosion of Metals by Milk’ from the January 1932 Journal of Dairy Science by Fink and Rohrman states: ‘It has long been known that milk in contact with iron and copper will not only acquire a metallic taste, but corrode these metals readily’. At that time, tin-coated metals were commonly used. It went on to say that ‘High chromium nickel (18-8) iron alloys … are very resistant to corrosion by milk and are satisfactory for dairy equipment …’. The modern milk processing industry is filled with stainless steel equipment, mostly of Type 304 (EN 1.4301) or 304L.
The report also went on to state that some materials that are otherwise suitable for processing of milk ‘…do not stand up well to the action of cleaning compounds that are commonly used in dairies’, but that the 18-8 alloy was suitable for those cleaning compounds. Today, the typical cleaning acids and hypochlorite sanitising compounds that are used not only in the dairy industry but also in most food and beverage plants worldwide, require that same 18-8 alloy as a minimum. A correctly chosen stainless steel alloy will not change the taste or appearance
of the food product. However, it is the ability to withstand repeated use of the sanitising chemicals over the lifetime of the equipment that has led to the widespread use of stainless steel in all sectors of the food and beverage industry. Producers are then able to guarantee the
safety of their food products.
 ARCHITECTURE
ARCHITECTURE
Another area of quick acceptance was in architecture. The first recorded use for that purpose was in 1929 in London at the Savoy Hotel where a sidewalk canopy and a sign were erected with the 18-8 alloy. These were soon followed by two iconic skyscrapers in New York that used stainless steel as a dominant element on their exteriors: the Chrysler Building in 1930 and the Empire State Building in 1931.
Since then, many prestigious buildings around the world have used stainless steel, including the Petronas Tower in Kuala Lumpur, the Trump Tower in Chicago, and the Jin Mao Tower in Shanghai. Related to architecture is sculpture, and Isamu Noguchi convinced the Associated Press in 1940 to approve stainless steel instead of bronze for his sculpture above the entrance to its building in New York. Since then, artists around the world have been using stainless steel, mostly either 304L or 316L (EN 1.4404), in their works. The St Louis Arch in the USA, Frank Gehry’s Peis (Fish) in Barcelona, Spain, and more recently Genghis Khan in Mongolia are examples of what can be done with stainless steel.
TRANSPORTATION
During the Great Depression in the USA, Edward Budd realised the untapped potential for stainless steels. Although their use in aeroplanes was his first application, his legacy remains the building of more than 10,000 passenger railcars, some of them still in use today.
Around the world, stainless steel is used extensively for passenger rail cars for subways, commuter trains and long distance trains, ensuring safety plus long life and low maintenance costs. In addition, stainless steels are used to transport cargoes such as food products, petroleum products and corrosive chemicals by rail, road, water and even air, both domestically and internationally.
ENERGY
In the broad field of energy, stainless steels have been used to extract oil and gas containing hazardous substances as well as for use in the refining stages. For power plants, stainless steel is used extensively at both low and high temperatures, whether the fuel is coal, oil, gas, uranium or waste products. Hydroelectric stations use stainless steel for dam gates as well as turbines. Many of the established sustainable
energy technologies such as solar and geothermal are using stainless steel, as well as the present biofuels industry with corn or sugar cane as feed stock.
WATER
Fresh water is an essential commodity for mankind, and stainless steel is used extensively in treatment plants for potable water as well as  for wastewater. Cost effectively producing fresh water from seawater or brackish water by desalination also requires the use of stainless steel. In some countries, underground stainless steel pipe is used to deliver potable water to homes to prevent leakage, or in other special cases to protect either the environment outside the pipe or the water inside the pipe. Stainless steel plumbing is also common in certain countries and offers a long lasting, low maintenance option.
for wastewater. Cost effectively producing fresh water from seawater or brackish water by desalination also requires the use of stainless steel. In some countries, underground stainless steel pipe is used to deliver potable water to homes to prevent leakage, or in other special cases to protect either the environment outside the pipe or the water inside the pipe. Stainless steel plumbing is also common in certain countries and offers a long lasting, low maintenance option.
SURGERY
The first recorded example of an austenitic stainless steel surgical implant is from 1926. Medical instruments are also known from that time period. The ability to easily and repeatedly sterilise components that come in contact with the human body or are used in hospitals and clinics contributed to the early acceptance of stainless steel. Today, there are well-established international specifications for materials used in this industry. For example, stainless steel alloys for implants must meet stringent metallurgical cleanliness requirements and be completely non-magnetic so that the patient can safely undergo diagnosis by Magnetic Resonance Imaging.
 FUTURE USES OF STAINLESS STEEL
FUTURE USES OF STAINLESS STEEL
Strong growth in the use of stainless steel has continued in the past decades despite the rapid and diverse developments in other materials and the more recent economic turmoil. The nickel-containing alloys in the 300 series still account for nearly two thirds of current stainless steel production worldwide, and there is nickel in the 200 series, duplex and precipitation hardening families, as well as in some of the martensitic and ferritic alloys. The reason for this is the great value that is placed on the properties which nickel provides.
Society is rapidly evolving and facing challenges on a global scale. Population is increasing, expectations are growing and resources are limited. Therefore we must use those resources more efficiently. This is particularly apparent for energy where stainless steel, and especially the nickel-containing alloys, already plays a major role in the more difficult to extract fossil fuels. Stainless steel’s corrosion and heat resisting properties are key to more cost-efficient operations. This also applies to the renewable sources that are now being developed, such as wave power and biofuels from new organic sources.
The worldwide need for higher quality, safe food and beverages and water will only increase, especially as food products can come from anywhere in the world. Stainless steel has evolved as the material of choice in this industry, both industrially and domestically, and it is likely to continue to meet the demands of a global population that is predicted to increase to nine billion by 2050.
This growing population, combined with a rapid movement to urbanisation, requires an expanded and more efficient transport infrastructure. The characteristics of stainless steel enable it to deliver lightweight and durable designs, leading to more efficient performance, safety, lower energy requirements and reduced emissions while giving lower life-cycle costs.
Image of Trump Tower (Chicago, USA) pictured above courtesy of C.Houska.
This article is featured in Australian Stainless magazine, issue 52.